

报告题目:Controlling the Energy Structure of AgInGaS Quantum Dots for Enhancing Their Photoluminescence Property and Photocatalytic Activity
报告时间:2022年9月23日上午10:00-11:30
报告地点:腾讯会议
会议ID: 578-702-581
报 告 人:Prof. Tsukasa Torimoto(教授、名古屋大学)
报告主持人:Prof. Masakazu Ampo(教授、红宝石hbs0022平台&日本大阪公立大学)
报告摘要:
Quantum dots (QDs) composed of group I-III-VI semiconductors, such as CuInS2 and AgInS2, exhibit unique size- and composition-dependent physicochemical properties different from bulk materials due to the quantum size effect. Furthermore, these QDs have attracted intense attention for developing highly efficient solar energy conversion systems, because of the less toxicity and the large absorption coefficients in visible and near-IR regions. Recently we have successfully prepared alloy QDs composed of ZnAgInS or ZnAgInSe semiconductors.(1,2) Their photocatalytic H2 evolution activity was controlled by the chemical composition and size of QDs. On the other hand, the Eg of bulk I-III-VI semiconductors can be controlled by alloying different metal cations of group III elements. In this lecture, I introduce the preparation of less-toxic AgIn(1-x)GaxS2 (AIGS(x)) alloy QDs and their photoluminescence (PL) property and photocatalytic activity for H2 evolution.
AIGS(x) QDs with different x values were synthesized by thermal decomposition of Ag(OAc), In(acac)3, Ga(acac)3, and S powders in a mixture solution of oleylamine and dodecanethiol (DDT). The composition of resulting QDs, that is, x value, was controlled by varying the Ga/(In+Ga) ratio in preparation. Thus-obtained AIGS QDs were surface-coated with GaSx or ZnS shells, resulting in the formation of core-shell-structured QDs, AIGS(x)@GaSx or AIGS(x)@ZnS. The surface modifiers on QDs were changed from DDT to mercaptopropionic acid (MPA) for photocatalytic H2 evolution in an aqueous solution.
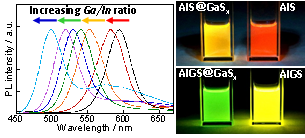
Fig. 1 PL spectra of AIGS@GaSx QDs and photographs of solutions containing AIGS and AIGS@GaSx QDs
The obtained AIGS(x) QDs had a spherical shape with size of ca. 4 nm. AIGS(x) QDs exhibited a sharp band-edge PL peak as well as a broad defect-site emission.(3) The peak wavelength of the band edge emission was tunable from 610 to 500 nm with an increase in the x value as a result of an increase of the Eg. The surface coating of AIGS(x) with GaSx shell drastically and selectively suppressed the broad defect-site PL peak (Fig. 1) and, at the same time, led to an increase in the PL quantum yield (QY) of the band edge emission peak. The optimal PL QY was 28% for AIGS(x)@GaSx QDs with green band-edge emission at 530 nm and a full width at half-maximum of 181 meV (41 nm).
ZnS shells of 0~ 2 nm in thickness were coated on AIGS(x) QDs for photocatalytic H2 evolution. The irradiation to AIGS(x)@ZnS QDs suspended in an aqueous solution containing Na2S as a hole scavenger was carried out using Xe lamp light (λ> 350 nm). With the elapse of light irradiation, the amount of H2 evolved linearly increased, regardless of the composition of AIGS core or the ZnS shell thickness. The H2 evolution rate was remarkably dependent on the composition of AIGS core. The highest photocatalytic activity was obtained for AIGS(x)@ZnS QDs with x=0.6 and the ZnS shell thickness of ca. 2 nm. These behavior can be explained by the changes in the stability of AIGS core in aqueous solutions, the conduction band level of QDs, and the amount of photon absorption.
References: (1) T. Kameyama, et al., J. Phys. Chem. C 2015, 119, 24740-24749. (3) P.-Y. Hsieh, et al., J. Mater. Chem. A 2020, 8, 13142-13149. (3) T. Kameyama, et al., ACS Appl. Mater. Interfaces 2018, 10, 42844−42855.
报告人简介:
Tsukasa Torimoto教授自2005起就职于日本名古屋大学(Nagoya University)。
获奖情况:
2017 日本电化学协会科学成就奖
2016 日本光化学协会奖
2001 日本电化学协会青年研究员奖